High pH instability of quaternary ammonium surfactant coatings in capillary electrophoresis†
Abstract
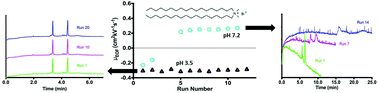
The two-tailed cationic surfactant dioctadecyldimethyl ammonium bromide (DODAB) produces semi-permanent coatings that yield strongly reversed electroosmotic flow (EOF), for example −0.31 ± 0.01 cm2 kV−1 s−1 at pH 3.5. Moreover, these coatings are easy to prepare, regenerable, cost effective, and yield high efficiency (520 000–900 000 plates per m) separations of cationic proteins over many runs under acidic (pH 3.5) conditions. Given the quaternary amine functionality of DODAB, we were surprised to observe that DODAB coatings become unstable at pH > 7. At pH 7.2, the EOF of a DODAB coated capillary drifted from reversed to cathodic over only 5 runs, and protein separations became severely compromised. By pH 12, no EOF reversal was observed. Electrophoretic and mass spectrometric studies demonstrate that the coating decomposition involves a surface conversion of the quaternary amine in DODAB to a variety of products, although the exact mechanism remains elusive. Regardless, the results herein demonstrate that semi-permanent coatings based on cationic two-tailed surfactants such as DODAB are limited to separations using acidic buffers.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...