Surfactant exfoliated 2D hexagonal Boron Nitride (2D-hBN) explored as a potential electrochemical sensor for dopamine: surfactants significantly influence sensor capabilities†
Abstract
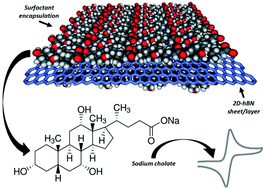
Surfactant exfoliated 2D hexagonal Boron Nitride (2D-hBN) nanosheets are explored as a potential electrochemical sensing platform and evaluated towards the electroanalytical sensing of dopamine (DA) in the presence of the common interferents, ascorbic acid (AA) and uric acid (UA). Surfactant exfoliated 2D-hBN nanosheets (2–4 layers) fabricated using sodium cholate in aqueous media are electrically wired via a drop-casting modification process onto disposable screen-printed graphite electrodes (SPEs). We critically evaluate the performance of these 2D-hBN modified SPEs and demonstrate the effect of ‘mass coverage’ towards the detection of DA, AA and UA. Previous studies utilising surfactant-free (pristine) 2D-hBN modified SPEs have shown a beneficial effect towards the detection of DA, AA and UA when compared to the underlying/unmodified graphite-based electrode. We show that the fabrication route utilised to prepare 2D-hBN is a vital experimental consideration, such that the beneficial effect previously reported is considerably reduced when surfactant exfoliated 2D-hBN is utilised. We demonstrate for the first time, through implementation of control experiments in the form of surfactant modified graphite electrodes, that sodium cholate is a major contributing factor to the aforementioned detrimental behaviour. The significance here is not in the material per se, but the fundamental knowledge of the surfactant and surface coverage changing the electrochemical properties of the material under investigation. Given the wide variety of ionic and non-ionic surfactants that are utilised in the manufacture of novel 2D materials, the control experiments reported herein need to be performed in order to de-convolute the electrochemical response and effectively evaluate the ‘underlying surface/surfactant/2D materials’ electrocatalytic contribution.
- This article is part of the themed collection: Analyst Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...