Microcontact printing with aminosilanes: creating biomolecule micro- and nanoarrays for multiplexed microfluidic bioassays†
Abstract
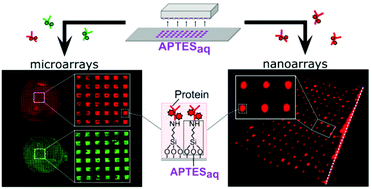
Microfluidic systems integrated with protein and DNA micro- and nanoarrays have been the most sought-after technologies to satisfy the growing demand for high-throughput disease diagnostics. As the sensitivity of these systems relies on the bio-functionalities of the patterned recognition biomolecules, the primary concern has been to develop simple technologies that enable biomolecule immobilization within microfluidic devices whilst preserving bio-functionalities. To address this concern, we introduce a two-step patterning approach to create micro- and nanoarrays of biomolecules within microfluidic devices. First, we introduce a simple aqueous based microcontact printing (μCP) method to pattern arrays of (3-aminopropyl)triethoxysilane (APTES) on glass substrates, with feature sizes ranging from a few hundred microns down to 200 nm (for the first time). Next, these substrates are integrated with microfluidic channels to then covalently couple DNA aptamers and antibodies with the micro- and nanopatterned APTES. As these biomolecules are covalently tethered to the device substrates, the resulting bonds enable them to withstand the high shear stresses originating from the flow in these devices. We further demonstrated the flexibility of this technique, by immobilizing multiple proteins onto these APTES-patterned substrates using liquid-dispensing robots to create multiple microarrays. Next, to validate the functionalities of these microfluidic biomolecule microarrays, we perform (i) aptamer-based sandwich immunoassays to detect human interleukin 6 (IL6); and (ii) antibody-based sandwich immunoassays to detect human c-reactive protein (hCRP) with the limit of detection at 5 nM, a level below the range required for clinical screening. Lastly, the shelf-life potential of these ready-to-use microfluidic microarray devices is validated by effectively functionalizing the patterns with biomolecules up to 3 months post-printing. In summary, with a single printing step, this aminosilane patterning technique enables the creation of functional microfluidic micro- and nano-biomolecule arrays, laying the foundation for high-throughput multiplexed bioassays.


 Please wait while we load your content...
Please wait while we load your content...