Detection of glycosylation and iron-binding protein modifications using Raman spectroscopy
Abstract
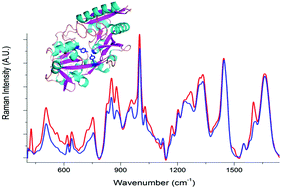
In this study we demonstrate the use of Raman spectroscopy to determine protein modifications as a result of glycosylation and iron binding. Most proteins undergo some modifications after translation which can directly affect protein function. Identifying these modifications is particularly important in the production of biotherapeutic agents as they can affect stability, immunogenicity and pharmacokinetics. However, post-translational modifications can often be difficult to detect with regard to the subtle structural changes they induce in proteins. From their Raman spectra apo-and holo-forms of iron-binding proteins, transferrin and ferritin, could be readily distinguished and variations in spectral features as a result of structural changes could also be determined. In particular, differences in solvent exposure of aromatic amino acids residues could be identified between the open and closed forms of the iron-binding proteins. Protein modifications as a result of glycosylation can be even more difficult to identify. Through the application of the chemometric techniques of principal component analysis and partial least squares regression variations in Raman spectral features as a result of glycosylation induced structural modifications could be identified. These were then used to distinguish between glycosylated and non-glycosylated transferrin and to measure the relative concentrations of the glycoprotein within a mixture of the native non-glycosylated protein.
- This article is part of the themed collection: Analyst Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...