Initial stages of CO2 adsorption on CaO: a combined experimental and computational study†
Abstract
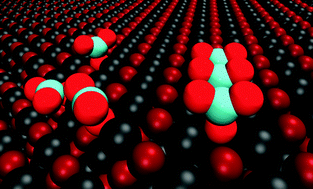
Room temperature adsorption of carbon dioxide (CO2) on monocrystalline CaO(001) thin films grown on a Mo(001) substrate was studied by infrared reflection–absorption spectroscopy (IRAS) and quantum chemical calculations. For comparison, CO2 adsorption was examined on poorly ordered, nanoparticulate CaO films prepared on Ru(0001). For both systems, CO2 readily adsorbs on the clean CaO surface. However, additional bands were observable on the CaO/Ru(0001) films compared with CaO/Mo(001), because the stricter IRAS surface selection rules do not apply to adsorption on the disordered thin films grown on Ru(0001). Spectral evolution with increasing exposure of the IRA bands suggested the presence of several adsorption sites which are consecutively populated by CO2. Density functional calculations showed that CO2 adsorption occurs as monodentate surface carbonate (CO32−) species at monatomic step sites and other low-coordinated sites, followed by formation of carbonates on terraces, which dominate at increasing CO2 exposure. To explain the coverage-dependent IRAS results, we propose CO2 surface islanding from the onset, most likely in the form of pairs and other chain-like species, which were calculated as thermodynamically favorable. The calculated adsorption energy for isolated CO2 on the terrace sites (184 ± 10 kJ mol−1) is larger than the adsorption energy obtained by temperature programmed desorption (∼120–140 kJ mol−1) and heat of adsorption taken from microcalorimetry measurements at low coverage (∼125 kJ mol−1). However, the calculated adsorption energies become less favorable when carbonate chains intersect on CaO terraces, forming kinks. Furthermore, our assignments of the initial stages of CO2 adsorption are consistent with the observed coverage effect on the CO2 adsorption energy measured by microcalorimetry and the IRAS results.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...