An in vitro and in vivo bio-interaction responses and biosafety evaluation of novel Au–ZnTe core–shell nanoparticles†
Abstract
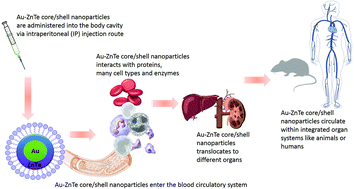
Novel gold–zinc telluride (Au–ZnTe) core–shell nanoparticles were synthesized to support surface modifications for enhanced drug delivery in cancer therapeutics. Knowledge of the biosafety and biocompatibility properties of these materials within biological systems is very limited and needs to be evaluated before their potential bio-applications may be demonstrated. We report the in vitro and in vivo bio-interactions of the Au–ZnTe nanoparticles, which were exposed to various human cancer and healthy cells, an in vitro immune simulation using peripheral blood mononuclear cells, followed by the analysis of cytokine expression. Acute in vivo exposure studies using low (50 μg ml−1), intermediate (500 μg ml−1) and high (1500 μg ml−1) concentrations of the Au–ZnTe particles were used to investigate histopathological effects in rats. Normal human mammary epithelial and colon cells in addition to human breast, prostate and colon cancer cells displayed cell viability between 86.4 ± 7.4% and 99.0 ± 3.6% when co-cultured with core–shell nanoparticles for 48 hours. Acute exposure studies using rat models displayed no significant changes in full blood counts, liver and kidney enzyme regulation and histopathology. These findings confirmed that Au–ZnTe core–shell nanoparticles display biosafety and biocompatibility features which can be exploited in future bio-applications.

 Please wait while we load your content...
Please wait while we load your content...