Synthesis, molecular and photovoltaic/transistor properties of heptacyclic ladder-type di(thienobenzo)fluorene-based copolymers†
Abstract
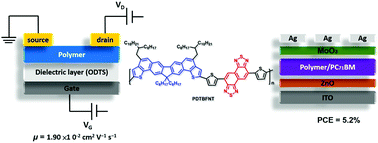
We present a facile synthesis method to make a new ladder-type heptacyclic dithienobenzofluorene (DTBF) framework, where the central 2,7-fluorene unit is covalently fastened with two external thiophenes via two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bridges. A dieneyne-containing precursor undergoes DBU-induced double benzannulation to regiospecifically introduce two solubilizing 2-octyldodecyl side chains at 5,10-positions of DTBF. The rigid and coplanar Br-DTBF monomer with sufficient solubility was copolymerized with 5,6-difluoro-4,7-bis(5-(trimethylstannyl)thiophen-2-yl)benzo[c][1,2,5]thiadiazole (Sn-DTFBT) and 5,10-bis(5-(trimethylstannyl)thiophen-2-yl)naphtho[1,2-c:5,6-c′]bis([1,2,5]thiadiazole) (Sn-DTNT) via Stille coupling to furnish two donor–acceptor copolymers, PDTBFFBT and PDTBFNT, respectively. Their thermal, optical, electrochemical, molecular stacking and photovoltaic properties are investigated. PDTBFNT has a higher molecular weight, smaller optical and electrochemical band gaps, and stronger solid-state packing than PDTBFFBT. DFT calculations were carried out to gain insight into the electronic and structural properties of DTBF and its derivatives. Bulk heterojunction solar devices with the ITO/ZnO/polymers:PC71BM/MoO3/Ag configuration were fabricated. By adding 5 vol% diphenyl ether (DPE) as an additive, PDTBFNT:PC71BM and PDTBFFBT:PC71BM devices achieved the power conversion efficiencies of 5.22% and 2.68%, respectively. The superior efficiency of PDTBFNT over PDTBFFBT is attributed to the better LUMO energy alignment between PDTBFNT and PC71BM and the face-on π-stacking of PDTBFNT in the active layer. Moreover, PDTBFNT exhibited a higher field-effect transistor hole mobility of 1.90 × 10−2 cm2 V−1 s−1 than PDTBFFBT with a value of 3.96 × 10−3 cm2 V−1 s−1.
C bridges. A dieneyne-containing precursor undergoes DBU-induced double benzannulation to regiospecifically introduce two solubilizing 2-octyldodecyl side chains at 5,10-positions of DTBF. The rigid and coplanar Br-DTBF monomer with sufficient solubility was copolymerized with 5,6-difluoro-4,7-bis(5-(trimethylstannyl)thiophen-2-yl)benzo[c][1,2,5]thiadiazole (Sn-DTFBT) and 5,10-bis(5-(trimethylstannyl)thiophen-2-yl)naphtho[1,2-c:5,6-c′]bis([1,2,5]thiadiazole) (Sn-DTNT) via Stille coupling to furnish two donor–acceptor copolymers, PDTBFFBT and PDTBFNT, respectively. Their thermal, optical, electrochemical, molecular stacking and photovoltaic properties are investigated. PDTBFNT has a higher molecular weight, smaller optical and electrochemical band gaps, and stronger solid-state packing than PDTBFFBT. DFT calculations were carried out to gain insight into the electronic and structural properties of DTBF and its derivatives. Bulk heterojunction solar devices with the ITO/ZnO/polymers:PC71BM/MoO3/Ag configuration were fabricated. By adding 5 vol% diphenyl ether (DPE) as an additive, PDTBFNT:PC71BM and PDTBFFBT:PC71BM devices achieved the power conversion efficiencies of 5.22% and 2.68%, respectively. The superior efficiency of PDTBFNT over PDTBFFBT is attributed to the better LUMO energy alignment between PDTBFNT and PC71BM and the face-on π-stacking of PDTBFNT in the active layer. Moreover, PDTBFNT exhibited a higher field-effect transistor hole mobility of 1.90 × 10−2 cm2 V−1 s−1 than PDTBFFBT with a value of 3.96 × 10−3 cm2 V−1 s−1.

 Please wait while we load your content...
Please wait while we load your content...