Insights into the correlation between the molecular conformational change and AIE activity of 2,5-bis(dimesitylboryl)-3,4-diphenylsiloles†
Abstract
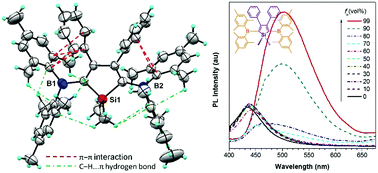
Three novel AIEgens with low-lying LUMO energy levels are developed from p-π conjugated 2,5-bis(dimesitylboryl)-3,4-diphenylsiloles. It is found that intramolecular interactions lower the molecular conformational changes, giving rise to broad 1H NMR peaks and decreased AIE activity.

 Please wait while we load your content...
Please wait while we load your content...