An ESIPT fluorophore with a switchable intramolecular hydrogen bond for applications in solid-state fluorochromism and white light generation†
Abstract
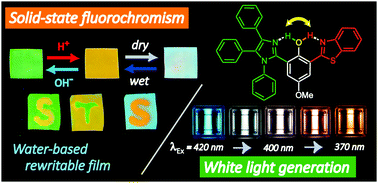
A novel excited state intramolecular proton transfer (ESIPT) fluorophore of BTImP, in which benzothiazole (BT) and imidazole (Im) rings are respectively linked to the 2,6 positions of a phenol, was designed and synthesized. The switching of two intramolecular hydrogen bonds from the phenol proton to either the BT or Im nitrogen was reversibly induced by external acid/base stimuli due to protonation/deprotonation of the Im site. The fluorescence color of BTImP was thus changed with high contrast between green and orange, which could be achieved even in a BTImP-doped Nafion film. Writing a letter on the film using acidic or basic water as ink was demonstrated. Furthermore, the property that the protonated state of BTImP is sensitive to the surrounding anions provides other possibilities not only for blue/orange fluorochromism in Nafion film by dry–wet treatments, but also for white light generation in solution by tuning of the excitation wavelength.

 Please wait while we load your content...
Please wait while we load your content...