‘Ferroelectric’ metals reexamined: fundamental mechanisms and design considerations for new materials†
Abstract
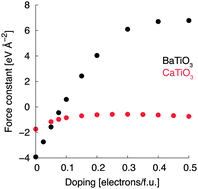
The recent observation of a ferroelectric-like structural transition in metallic LiOsO3 has generated a flurry of interest in the properties of polar metals. Such materials are thought to be rare because free electrons screen out the long-range electrostatic forces that favor a polar structure with a dipole moment in every unit cell. In this work, we question whether long-range electrostatic forces are always the most important ingredient in driving polar distortions. We use crystal chemical models, in combination with first-principles Density Functional Theory calculations, to explore the mechanisms of inversion-symmetry breaking in LiOsO3 and both insulating and electron-doped ATiO3 perovskites, A = Ba, Sr, Ca. Although electrostatic forces do play a significant role in driving the polar instability of BaTiO3 (which is suppressed under electron doping), the polar phases of CaTiO3 and LiOsO3 emerge through a mechanism driven by local bonding preferences and this mechanism is ‘resistant’ to the presence of charge carriers. Hence, our results suggest that there is no fundamental incompatibility between metallicity and polar distortions. We use the insights gained from our calculations to suggest design principles for new polar metals and promising avenues for further research.
- This article is part of the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials

 Please wait while we load your content...
Please wait while we load your content...