Triplet excited state of diiodoBOPHY derivatives: preparation, study of photophysical properties and application in triplet–triplet annihilation upconversion†
Abstract
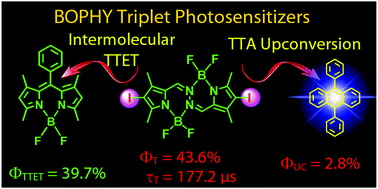
A pyrrole-BF2-based chromophore bis(difluoroboron)1,2-bis((pyrrol-2-yl)methylene) hydrazine (BOPHY) was used for the first time for the preparation of new triplet photosensitizers. The UV-vis absorption spectra show that the absorption bands of the photosensitizers cover the 400–700 nm range. Nanosecond time-resolved transient absorption spectra show that the triplet excited state of the compounds was populated upon photoexcitation and the compound diiodoBOPHY (C-2) is with a long triplet excited state lifetime of 177.2 μs. The triplet state energy level of C-2 was demonstrated to be higher than the traditional BODIPY chromophores. DFT/TDDFT calculations were carried out for rationalization of the photophysical properties of the compounds. C-2 was used in triplet–triplet annihilation upconversion, using 9,10-diphenylanthracene (DPA) as the triplet energy acceptor. The TTA upconversion quantum yield is 2.8%. With the styryl substituents on the BOPHY core, the sensitizers demonstrated lower triplet state energy levels and shorter triplet state lifetimes. Using these triplet photosensitizers, photooxidation of 1,3-diphenylisobenzofuran (DPBF) by singlet oxygen (1O2) photosensitization was also carried out.

 Please wait while we load your content...
Please wait while we load your content...