Unimolecular micelles of camptothecin-bonded hyperbranched star copolymers via β-thiopropionate linkage: synthesis and drug delivery†
Abstract
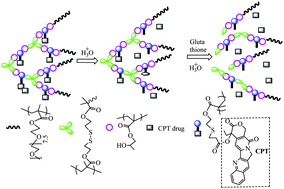
In order to develop pH- and redox-responsive unimolecular micelles composed of camptothecin (CPT)-conjugated hyperbranched star copolymers via acid-labile β-thiopropionate linkage, a new monomer, methacryloyloxy-3-thiohexanoyl–CPT, is synthesized through conjugation of CPT with methacrylate via β-thiopropionate linkage, and then used in synthesis of the CPT-conjugated hyperbranched star copolymers by two steps of atom transfer radical polymerization (ATRP): self-condensation vinyl polymerization of the CPT-based monomer, 2-hydroxypropyl methacrylate and inimer, and subsequent ATRP of oligo(ethylene glycol) methacrylate using the obtained hyperbranched polymers as the macroinitiator. The obtained polymers dissolve in water to form unimolecular micelles, and their release of CPT in water at various pHs and their anticancer efficacy are studied. The CPT-loaded unimolecular micelles with diameters of 3.56–6.08 nm are quite stable under neutral environment, and are easily triggered by mild acidic pH, such as 6.0 and 5.0. They can be easily internalized by the tumor cells, releasing the CPT. The CPT-conjugated unimolecular micelles via acid-labile β-thiopropionate linkage have potential for application as tumor-targeted drug release systems.

 Please wait while we load your content...
Please wait while we load your content...