Rational design and synthesis of LiTi2(PO4)3−xFx anode materials for high-performance aqueous lithium ion batteries†
Abstract
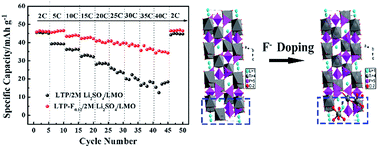
Aqueous lithium ion batteries have shown advantages of high safety and low cost, because of the use of nontoxic and nonflammable aqueous electrolytes. LiTi2(PO4)3/LiMn2O4 is considered as one of the most promising aqueous lithium ion battery systems for its moderate working voltage, and high electrochemical stability in aqueous electrolytes. However, the critical issue of hydrogen evolution during the charge process hinders its development. In this paper, F− was first introduced in LiTi2(PO4)3 to raise the ion intercalation potential and further to solve the hydrogen evolution problem. Besides, F− doping can decrease the band gap and further increase the intrinsic electronic conductivity. Additionally, the diffusion coefficient of Li+ increased by one order of magnitude after F− doping. Combined with the elevated potential and high conductivity, F-doped LiTi2(PO4)3 exhibited excellent cycling ability and rate performance. As a result, the power density and energy density of LiTi2(PO4)2.88F0.12/LiMn2O4 full cells reach 2794 W kg−1 and 43.7 W h kg−1 respectively, which are among the highest values ever reported for aqueous lithium or sodium ion batteries. The F− doping strategy was demonstrated to be a facile and effective method to fabricate anode materials for high-performance aqueous lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...