Nickel-titanium oxide as a novel anode material for rechargeable sodium-ion batteries†
Abstract
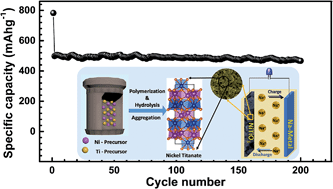
Nickel-titanium oxide (NiTiO3; NTO) of an ilmenite structure that comprises a layered transition-metal octahedral structure, wherein the zigzag open tunnels are possible routes for Na intercalation, can be a potential anode material for sodium (Na) ion batteries (SIBs). In this study, nanocrystalline NTO particles that are of sizes 3 to 5 nm were prepared using a simple hydrothermal process followed by annealing, and the particles were then tested for SIB applications. The pure-NTO electrode that comprises a hexagonal crystal structure and mesoporous morphology demonstrated a reversible capacity of approximately 521 mA h g−1 that corresponds to a coulombic efficiency of 67% in the first cycle, which further improved to ∼98% in the following cycles, at an applied specific current of 50 mA g−1, and stable cycling performance for 200 cycles. Further, due to the synergetic effect of the porous network structure and high surface area, the NTO electrode exhibited an exceptional rate capability, delivering a capacity of 192 mA h g−1 at a high specific current of 4000 mA g−1. The excellent cyclability and rate capability of the NTO electrode are attributed to the improved electronic conductivity and highly porous microstructure of the NTO material, whereby fast charge transfer and facile diffusion of the Na-ions to the active sites are enabled.
- This article is part of the themed collection: 2016 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...