Framework-mediated synthesis of highly microporous onion-like carbon: energy enhancement in supercapacitors without compromising power†
Abstract
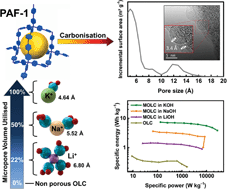
High power technologies require the delivery of large amounts of energy in brief time periods – a demand that cannot be met by the current materials used in supercapacitors because they do not store charge efficiently at high discharge rates. To address this unmet challenge, high surface area graphitic carbons with a large fraction of micropores, for increased capacity, and high pore connectivity in addition to high conductivity, for fast current-switching response, are needed. Typically, the high temperatures required for the transformation of carbonaceous precursors into graphitic networks collapses the micropores, so that even the most promising morphology in a precursor is lost during carbonisation. Here we demonstrate that a high surface area diamondoid framework (PAF-1) under moderate temperatures (≤900 °C) undergoes topology-preserved conversion into a onion-like carbon (OLC) material which uniquely exhibits high specific surface area (1084 m2 g−1), narrow, predominantly 5 Å pores and high electrical conductivity (2.8 S cm−1) – we term this as microporous onion-like carbon (MOLC). We measure capacitance of 211 F g−1 at 5 mV s−1, and unprecedented 52% capacitance retention at ultrafast 2000 mV s−1 – this property is a direct consequence of the curved graphitic planes of the OLCs incorporating microporosity from the precursor structure. Despite the fact that the micropores in our OLC are small and narrow, we show that optimal matching of the electrolyte (on the basis of solvated ionic radii) to the pore size enables us to achieve high power and energy densities simultaneously (—e.g., 3.85 W h kg−1 can be extracted at 27.7 kW kg−1). Also, MOLC electrodes with high-mass loading (8 mg cm−2) demonstrate remarkable areal capacitances (≈3 F cm−2) over a varied range of high discharge currents, which is among the highest reported for supercapacitor electrodes.

 Please wait while we load your content...
Please wait while we load your content...