Efficient and selective heavy metal sequestration from water by using layered sulfide K2xSn4−xS8−x (x = 0.65–1; KTS-3)†
Abstract
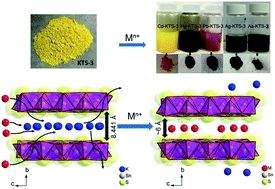
Heavy metal ions (Cd2+, Hg2+, As3+ and Pb2+) are an important contributor to the contamination of groundwater and other water bodies in and around industrial areas. Herein, we demonstrate the rapid and efficient capacity of a layered metal sulfide material, K2xSn4−xS8−x (x = 0.65–1, KTS-3) for heavy metal ion removal from water. The effect of concentration, pH, kinetics, and competitive ions such as Na+/Ca2+ on the heavy metal ion removal capacity of KTS-3 was systematically investigated. X-ray photoelectron spectroscopy (XPS), elemental analyses, and powder X-ray diffraction studies revealed that the heavy metal ion-exchange of KTS-3 is complete (quantitative replacement of all potassium ions) and topotactic. The heavy metal ion-exchange by using KTS-3 follows the Langmuir–Freundlich model with high exchange capacities, qm 205(17) mg g−1 for Cd2+, 372(21) mg g−1 for Hg2+ and 391(89) mg g−1 for Pb2+. KTS-3 retains excellent heavy metal ion-exchange capacity even in very high concentration (1 M) of competing ions (Na+/Ca2+) and also over a broad pH range (2–12). KTS-3 also exhibits very good ion-exchange capacity for precious Ag+ and toxic As3+ ions. The kinetics of heavy metal ion adsorption by KTS-3 are rapid (absorbs all ions within a few minutes). These properties and the environmentally friendly character of KTS-3 make it a promising candidate for sequestration of heavy metal ions from water.

 Please wait while we load your content...
Please wait while we load your content...