Significant reduction in the operating temperature of the Mn(ii)/Mn(iii) oxide-based thermochemical water splitting cycle brought about by the use of nanoparticles†
Abstract
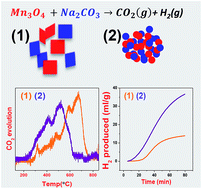
Among the many efforts to devise thermochemical cycles to generate H2 by water splitting, the Mn(II)/Mn(III) oxide based cycle operating at 850 °C is a significant one and involves no toxic and corrosive materials. The essential process in this cycle is the shuttling of Na+ ions in and out of Mn oxides. In an effort to bring down the temperature of this cycle, we have found that the use of Mn3O4 nanoparticles is particularly effective. Ball milling has been applied to decrease the particle size of commercial Mn3O4 to less than 500 nm. Thus the solid state reaction between Na2CO3 and Mn3O4 nanoparticles occurs at a temperature 200 °C lower than with bulk samples. One of the challenges of this particular cycle lies in its slow H2 evolution. It has been possible to operate this cycle and generate H2 at a much faster rate at 750 °C and even at 700 °C by this means. Furthermore, the step involving hydrolysis of NaMnO2 can be performed at 50 °C instead of 100 °C.

 Please wait while we load your content...
Please wait while we load your content...