Cs+ incorporation into CH3NH3PbI3 perovskite: substitution limit and stability enhancement†
Abstract
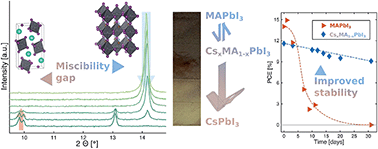
In this study we systematically explored the mixed cation perovskite Csx(CH3NH3)1−xPbI3. We exchanged the A-site cation by dipping MAPbI3 films into a CsI solution, thereby incrementally replacing the MA+ in a time-resolved dipping process and analysed the resulting thin-films with UV-Vis, XRD, EDAX, SEM and optical depth-analysis in a high-throughput fashion. Additional in situ UV-Vis and time-resolved XRD measurements allowed us to look at the kinetics of the formation process. The results showed a discontinuity during the conversion. Firstly, small amounts of Cs+ are incorporated into the structure. After a few minutes, the Cs content approaches a limit and grains of δ-CsPbI3 occur, indicating a substitution limit. We compared this cation exchange to a one-step crystallisation approach and found the same effect of phase segregation, which shows that the substitution limit is an intrinsic feature rather than a kinetic effect. Optical and structural properties changed continuously for small Cs incorporations. Larger amounts of Cs result in phase segregation. We estimate the substitution limit of CsxMA1−xPbI3 to start at a Cs ratio x = 0.13, based on combined measurements of EDAX, UV-Vis and XRD. The photovoltaic performance of the mixed cation perovskite shows a large increase in device stability from days to weeks. The initial efficiency of mixed CsxMA1−xPbI3 devices decreases slightly, which is compensated by stability after a few days.


 Please wait while we load your content...
Please wait while we load your content...