Phase separation synthesis of trinickel monophosphide porous hollow nanospheres for efficient hydrogen evolution†
Abstract
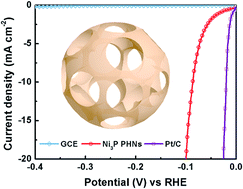
A facile and scalable approach to synthesize trinickel monophosphide (Ni3P) porous hollow nanospheres (PHNs) has been developed, the resultant Ni3P PHNs exhibiting excellent catalytic activity in the hydrogen evolution reaction (HER). The formation of the Ni3P PHNs correlates with phase separation during the thermal annealing of amorphous nickel–phosphorus nanospheres that affords crystalline Ni–Ni3P nanoparticles, and the subsequent selective removal of nickel. The overpotential required for the current density of 20 mA cm−2 is as small as 99 mV in acidic solution. The performance compares favorably with that of other metal phosphides, and is superior to that of transition metal dichalcogenides, carbides, borides, and nitrides. The faradaic efficiency of the Ni3P PHNs is 96%, and the Ni3P PHNs are stable during the long-term electrolysis of water. Density functional theory calculations suggest that a Ni–Ni bridge site and the sites on the top of the P atoms are the active sites during the HER. The scalable production, low cost, excellent catalytic activity, and long-term stability suggest promising application potential for Ni3P PHNs.

 Please wait while we load your content...
Please wait while we load your content...