Shale-like Co3O4 for high performance lithium/sodium ion batteries†
Abstract
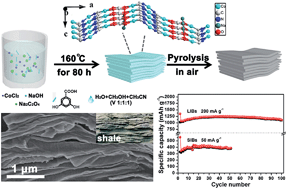
In recent years, metal-organic compounds have been considered as ideal sacrificial templates to obtain transition metal oxides for electrochemical applications due to their diverse structures and tunable properties. In this work, a new kind of cobalt-based metal organic compound with a layered structure was designed and prepared, which was then transformed into ultrafine cobalt oxide (Co3O4) nanocrystallites via a facile annealing treatment. The obtained Co3O4 nanocrystallites further assembled into a hierarchical shale-like structure, donating extremely short ion diffusion pathway and rich porosity to the materials. The special structure largely alleviated the problems of Co3O4 such as inferior intrinsic electrical conductivity, poor ion transport kinetics and large volume changes during the redox reactions. When evaluated as anode materials for lithium-ion batteries, the shale-like Co3O4 (S-Co3O4) exhibited superior lithium storage properties with a high capacity of 1045.3 mA h g−1 after 100 cycles at 200 mA g−1 and good rate capabilities up to 10 A g−1. Moreover, the S-Co3O4 showed decent electrochemical performance in sodium-ion batteries due to the above-mentioned comprehensive merits (380 and 153.8 mA h g−1 at 50 and 5000 mA g−1, respectively).

 Please wait while we load your content...
Please wait while we load your content...