Mo2C as a high capacity anode material: a first-principles study
Abstract
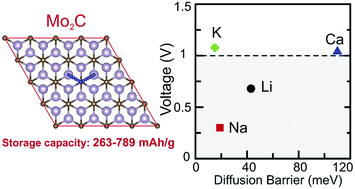
The adsorption and diffusion of Li, Na, K and Ca atoms on a Mo2C monolayer are systematically investigated by using first principles methods. We found that the considered metal atoms are strongly bound to the Mo2C monolayer. However, the adsorption energies of these alkali and earth alkali elements decrease as the coverage increases due to the enhanced repulsion between the metal ions. We predict a significant charge transfer from the ad-atoms to the Mo2C monolayer, which indicates clearly the cationic state of the metal atoms. The metallic character of both pristine and doped Mo2C ensures a good electronic conduction that is essential for an optimal anode material. Low migration energy barriers are predicted as small as 43 meV for Li, 19 meV for Na and 15 meV for K, which result in the very fast diffusion of these atoms on Mo2C. For Mo2C, we found a storage capacity larger than 400 mA h g−1 by the inclusion of multilayer adsorption. Mo2C expands slightly upon deposition of Li and Na even at high concentrations, which ensures the good cyclic stability of the atomic layer. The calculated average voltage of 0.68 V for Li and 0.30 V for Na ions makes Mo2C attractive for low charging voltage applications.
- This article is part of the themed collection: JMC A Editor’s choice collection: Recent advances in batteries

 Please wait while we load your content...
Please wait while we load your content...