Nanoporous Mn-based electrocatalysts through thermal conversion of cyano-bridged coordination polymers toward ultra-high efficiency hydrogen peroxide production†
Abstract
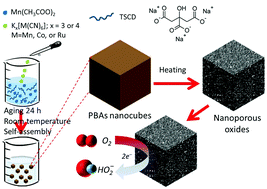
An oriented and controlled crystal growth of cyano-bridged coordination polymers is realized by a controlling agent (e.g., trisodium citrate dihydrate (TSCD)) as a reaction precursor. In the presence of TSCD, the reaction is slow as the complex appears to be more stable, leading to a preferentially oriented crystal growth. For instance, after mixing manganese acetate with TSCD, the formed Mn–citrate complex tends to release few Mn2+ ions steadily and slowly, which then react with the ligands at the initial stage of the reaction. Subsequently, the generated nuclei further grow from the interaction between the released Mn2+ and [Mn(CN)6]3−, [Co(CN)6]3−, or [Ru(CN)6]4− anions to form several types of cyano-bridged coordination polymers (abbreviated as MnCNMn, MnCNCo, or MnCNRu, respectively). After thermal treatment in air, the as-prepared coordination polymers can be decomposed into their corresponding nanoporous Mn-based oxides. Surprisingly, the electrochemical analysis reveals that the Mn–Ru oxide prepared from MnCNRu is a promising catalyst for the production of H2O2 by selectively catalyzing the oxygen reduction reaction (ORR) through an exact 2-electron pathway. Compared to previously reported materials, our electrocatalyst demonstrates an outstanding activity, a strict selectivity, and a long-term stability for the production of H2O2. The present catalyst design sheds new light on the production of H2O2 by a safe and sustainable way.


 Please wait while we load your content...
Please wait while we load your content...