Design and synthesis of an efficient nanoporous adsorbent for Hg2+ and Pb2+ ions in water†
Abstract
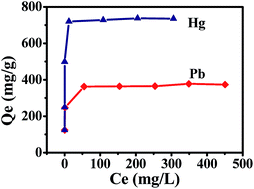
An efficient nanoporous adsorbent for Hg2+ and Pb2+ ions in water has been synthesized from the interaction of aqueous NaH2C3N3S3 and Na3C3N3S3 solutions. The Raman spectra of the sample indicate that the S–S bonds have been formed by this interaction, and N2 sorption isotherms together with TEM images indicate the presence of hierarchical nanopores (mesopores/macropores). Interestingly, this nanoporous sample exhibits excellent adsorption behavior of heavy metal ions in water, giving the adsorption capacity of Hg2+ and Pb2+ at 735.3 and 375.9 mg g−1, respectively. The Hg2+ and Pb2+ sorption isotherms are typical Langmuir and their kinetic model follows the pseudo-second-order. These results indicate that the adsorption behavior of the Hg2+ and Pb2+ ions on the sample is chemical. More importantly, this sample can be recycled 4 times without obvious loss of the adsorption ability in water, which is very important for its industrial applications in the future.

 Please wait while we load your content...
Please wait while we load your content...