Ferroelectric oxide surface chemistry: water splitting via pyroelectricity†
Abstract
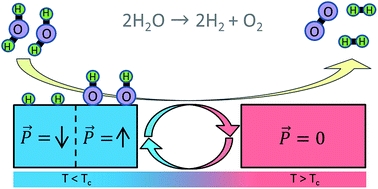
There is widespread interest in finding sustainable replacements for fossil fuels, and hence hydrogen production via water splitting has received much attention. However, finding efficient water splitting methods remains a challenge. We use first principles theory to design a catalytic cycle for water splitting that employs the pyroelectric effect in ferroelectrics. Taking PbTiO3 as an example, we show that actively controlling the ferroelectric polarization via cyclic temperature modulations can catalyze the splitting of H2O into O2 and H2. In practice, the energy needed to drive this cycle may be provided by low/intermediate grade heat. Since no precious metals are used in this catalytic cycle, this approach may lead to viable water-splitting methods that employ earth-abundant elements.

 Please wait while we load your content...
Please wait while we load your content...