Mesoporous Cr2O3 nanotubes as an efficient catalyst for Li–O2 batteries with low charge potential and enhanced cyclic performance†
Abstract
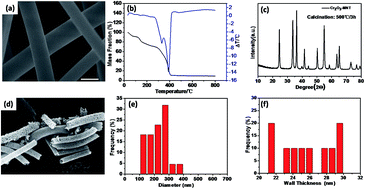
Hexagonal close packed Cr2O3, fabricated by an electrospinning technique combined with a heating method, is adopted for the first time as a catalyst for non-aqueous lithium–oxygen (Li–O2) batteries. The synthesized highly mesoporous Cr2O3 nanotubes (Cr2O3-MNT) with a large surface area of 53.4 m2 g−1 are confirmed by field emission scanning electron microscopy (FESEM), field emission high-resolution transmission electron microscopy (TEM) and nitrogen adsorption/desorption isotherms (BET). By using the prepared Super P (SP) (60 wt%)/Cr2O3 (30 wt%)/polyvinylidenefluoride (PVDF) (10 wt%) composite as an oxygen electrode, the Li–O2 battery shows an astonishingly enhanced capacity of 8280 mA h g−1 at a current density of 50 mA g−1. More encouragingly, when the current densities are fixed at 25, 50, 100 and 200 mA g−1 with a limited capacity of 500 mA h g−1, the charging potentials are 3.47, 3.51, 3.78 and 4.01 V, respectively, which are among the lowest charge potentials reported to date. By using a capacity-controlled method (1000 mA h g−1) at a current density of 100 mA g−1, the cell shows excellent cyclic stability up to 50 cycles. The reversible formation and dissociation of Li2O2 are verified by X-ray diffusion (XRD) and SEM, indicating that the as-prepared Cr2O3 nanotubes are a promising catalyst for Li–O2 batteries.

 Please wait while we load your content...
Please wait while we load your content...