Guest–host modulation of multi-metallic (oxy)hydroxides for superb water oxidation†
Abstract
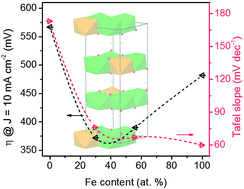
Multi-metallic (oxy)hydroxide materials are some of the most promising alternatives for superb water oxidation electrocatalysts. Herein, a series of graphene/NiFe (oxy)hydroxides were fabricated to investigate the role of the Fe/Ni ratio in regard to the resultant physical structures, decoration styles, and combined catalytic activities. With the Fe content increasing, a phase evolution from Fe doped Ni(OH)2/NiO(OH) to Ni doped FeO(OH) was demonstrated. The moderate guest-metal substitution into the host-oxyhydroxide framework (Fe into Ni or Ni into Fe) substantially enhanced the oxygen evolution activity with a decrease in both the Tafel slope and overpotential. In addition, the NiO(OH) and FeO(OH) frameworks exhibited distinct properties and performances for the oxygen evolution reaction due to the different metal–oxygen bond lengths and adsorption energies of the intermediates.

 Please wait while we load your content...
Please wait while we load your content...