Spontaneously polarized lithium-doped zinc oxide nanowires as photoanodes for electrical water splitting†
Abstract
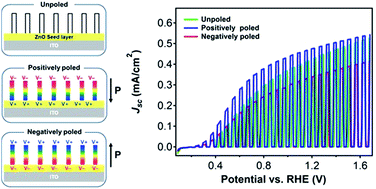
The key requirement for efficient water splitting is to form suitable band bending at the interface between the semiconductor and electrolyte. One intuitive approach is to directly induce dipoles inside photoelectrochemical cells (PECs) to render band bending favorable for water splitting. Ferroelectric materials, exhibiting spontaneous polarization, can be one promising material choice for this purpose as their polarization domains can be aligned in the desired direction by a poling process. In this work, we employed ferroelectric phase transformed Li-doped ZnO nanowires (NWs) as photoanodes for photoelectrochemical (PEC) water splitting and systematically investigated poling effects. Spontaneous polarization was induced in the NWs in the direction favorable for hole transfer to electrolytes, where the valence band bends upward at the electrolyte interface. Specifically, positively polarized PEC electrodes demonstrated ∼200% improved STH efficiency compared with negatively polarized ones.

 Please wait while we load your content...
Please wait while we load your content...