First row transition metal catalysts for solar-driven water oxidation produced by electrodeposition
Abstract
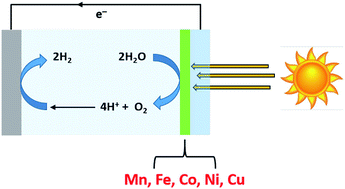
As our reliance on renewable energy resources increases, so will our need to store this energy in the form of chemical fuels to iron-out peaks and troughs in supply. Sunlight, the most plentiful source of renewable energy, is especially problematic in this regard as it is so diffuse. One way to convert solar irradiation to fuels effectively would be to develop large surface area photo-electrochemical devices that could use sunlight directly to split water into H2 and O2. However, in order to be feasible, such an approach requires that these devices (and their components) are extremely cheap. In this review, we will discuss catalysts for the water oxidation half-reaction of electrochemical water splitting that can be produced by electrodeposition (a technique well suited to large-scale, low-cost applications), and that are based on the comparatively plentiful and inexpensive first row transition metals. Special attention will be paid to the electrodeposition conditions used in the various examples given, and structure–function relationships for electrochemical water oxidation for the materials produced by these techniques will be elucidated.
- This article is part of the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials

 Please wait while we load your content...
Please wait while we load your content...