A new method for the synthesis of β-cyano substituted porphyrins and their use as sensitizers in photoelectrochemical devices†
Abstract
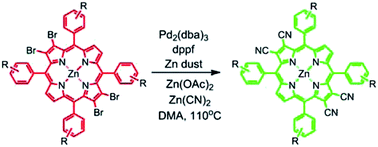
β-Cyanoporphyrins have high positive potentials for oxidation and absorb light at longer wavelengths than most porphyrins, making them potential candidates for sensitizers in photoelectrosynthetic cells for water oxidation. In order to begin to evaluate this potential, two Zn(II) tetra-β-cyanoporphyrins have been synthesized and evaluated as sensitizers in dye sensitized solar cells using I−/I3− as the redox mediator. To prepare such specialized β-cyanoporphyrins, a new synthetic method has been developed. This approach involves reaction of Zn(CN)2 with β-brominated zinc porphyrins in the presence of tris-(dibenzylideneacetone)dipalladium. The tetra-cyanation reaction is complete under milder conditions as compared to those usually employed in previous methods and gives improved yields of up to ∼50%. The procedure allows for the cyanation of porphyrins with relatively sensitive functional groups. Examples of its application to a range of substituted tetra-arylporphyrins are reported, and the absorption and electrochemical properties of the compounds prepared are given. The results from using two of the molecules as sensitizers in dye sensitized solar cells are presented. It was found that the porphyrins produced no photocurrents in nanoparticulate TiO2-based cells, but both molecules produced photocurrents in SnO2-based cells, and are potential candidates for sensitizers in photoelectrosynthetic cells for water oxidation.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...