Theoretical and experimental studies of highly active graphene nanosheets to determine catalytic nitrogen sites responsible for the oxygen reduction reaction in alkaline media†
Abstract
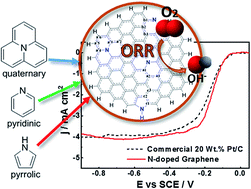
Graphene nanosheets with heterogeneously doped nitrogen atoms are synthesized using a facile one-step method based on extremely rapid heating (temperature ramps ≥ 150 °C s−1). Raman and X-ray photoelectron spectroscopy reveal efficacious nitrogen incorporation into the graphitic network. A half-cell testing conducted in alkaline media with a rotating disk electrode technique shows that the N-doped graphene nanosheets present a very comparable oxygen reduction reaction (ORR) activity to the state-of-the-art commercial 20 wt% Pt/C catalyst. The outstanding catalytic activity of metal-free carbon-based graphene nanosheets is ascribed to the opened structure produced by the facile one-step synthesis procedure resulting in significantly enhanced active site exposure. Density functional theory is used to elucidate the electrochemical properties of these nanosheets on the basis of bandgap, vertical ionization energies, and adsorption free energies of intermediate species (˙OOH, 3O, ˙OH, and ˙H) formed during the ORR associative mechanism occurring in alkaline media. A systematic analysis is conducted by comparing pristine graphene and N-doped graphene nanosheets of multiple sizes containing single and all together pyridinic, pyrrolic and graphitic species to determine the adequate size of the cluster and the most catalytically active N site to perform the ORR. From all possible adsorption sites for the intermediates including nitrogen on a larger cluster model with 37 rings doped with all types of nitrogen bonding, the adsorption on top of any carbon adjacent to a central graphitic nitrogen represents a significant reduction in the energy barrier of the ORR electrocatalysis. Theoretical calculations of the bond strengths of all possible species show that the removal of 3O species from the electrode surface to form OHads is the rate-controlling step of the ORR mechanism. Consistently, a surface electrode potential of 0.918 V vs. SHE computed for this reaction step is close to 0.867 V vs. SHE reported for the second reaction (OOH−/OH−) of the mechanism with two consecutive 2-electron transfer steps obtained under standard conditions.

 Please wait while we load your content...
Please wait while we load your content...