Membrane negative curvature induced by a hybrid peptide from pediocin PA-1 and plantaricin 149 as revealed by atomistic molecular dynamics simulations†
Abstract
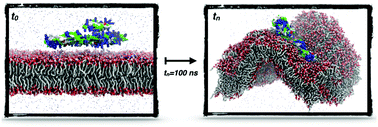
Antimicrobial peptides (AMPs) are cationic peptides that kill bacteria with a broad spectrum of action, low toxicity to mammalian cells and exceptionally low rates of bacterial resistance. These features have led to considerable efforts in developing AMPs as an alternative antibacterial therapy. In vitro studies have shown that AMPs interfere with membrane bilayer integrity via several possible mechanisms, which are not entirely understood. We have performed the synthesis, membrane lysis measurements, and biophysical characterization of a novel hybrid peptide. These measurements show that PA–Pln149 does not form nanopores, but instead promotes membrane rupture. It causes fast rupture of the bacterial model membrane (POPG-rich) at concentrations 100-fold lower than that required for the disruption of mammalian model membranes (POPC-rich). Atomistic molecular dynamics (MD) simulations were performed for single and multiple copies of PA–Pln149 in the presence of mixed and pure POPC/POPG bilayers to investigate the concentration-dependent membrane disruption by the hybrid peptide. These simulations reproduced the experimental trend and provided a potential mechanism of action for PA–Pln149. It shows that the PA–Pln149 does not form nanopores, but instead promotes membrane destabilization through peptide aggregation and induction of membrane negative curvature with the collapse of the lamellar arrangement. The sequence of events depicted for PA–Pln149 may offer insights into the mechanism of action of AMPs previously shown to induce negative deformation of membrane curvature and often associated with peptide translocation via non-bilayer intermediate structures.


 Please wait while we load your content...
Please wait while we load your content...