Force spectroscopy predicts thermal stability of immobilized proteins by measuring microbead mechanics†
Abstract
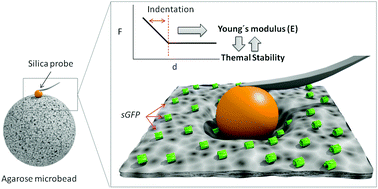
Optimal immobilization of enzymes on porous microbeads enables the fabrication of highly active and stable heterogeneous biocatalysts to implement biocatalysis in synthetic and analytical chemistry. However, empirical procedures for enzyme immobilization still prevail over rational ones because there is an unmet need for more comprehensive characterization techniques that aid to understand and trace the immobilization process. Here, we present the use of atomic force spectroscopy (AFS) as an innovative solution to indirectly characterize immobilized proteins on porous materials and monitor the immobilization process in real time. We investigate the mechanical properties of porous agarose microbeads immobilizing proteins by indenting a colloidal probe (silica microparticle) into a single bead. AFS demonstrates that the binding of proteins to the solid matrix of an agarose microbead alters its stiffness. Interestingly, we discovered that irreversible and multivalent immobilizations that make microbeads stiffer also stabilize the immobilized proteins against the temperature. Hence, we propose atomic force spectroscopy as a useful technique to indirectly unravel the stability of the immobilized enzymes investigating the mechanics of the heterogenous biocatalysts as a solid biomaterial beyond the intrinsic mechanics of the proteins.

 Please wait while we load your content...
Please wait while we load your content...