Osmotically-induced tension and the binding of N-BAR protein to lipid vesicles†
Abstract
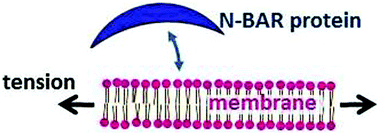
The binding affinity of a curvature-sensing protein domain (N-BAR) is measured as a function of applied osmotic stress while the membrane curvature is nearly constant. Varying the osmotic stress allows us to control membrane tension, which provides a probe of the mechanism of binding. We study the N-BAR domain of the Drosophila amphiphysin and monitor its binding on 50 nm-radius vesicles composed of 90 mol% DOPC and 10 mol% PIP. We find that the bound fraction of N-BAR is enhanced by a factor of approximately 6.5 when the tension increases from zero to 2.6 mN m−1. This tension-induced response can be explained by the hydrophobic insertion mechanism. From the data we extract a hydrophobic domain area that is consistent with known structure. These results indicate that membrane stress and strain could play a major role in the previously reported curvature-affinity of N-BAR.

 Please wait while we load your content...
Please wait while we load your content...