Contact electrification induced interfacial reactions and direct electrochemical nanoimprint lithography in n-type gallium arsenate wafer†
Abstract
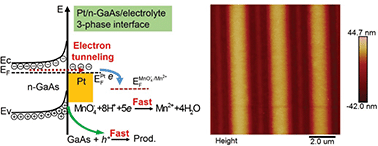
Although metal assisted chemical etching (MacEtch) has emerged as a versatile micro-nanofabrication method for semiconductors, the chemical mechanism remains ambiguous in terms of both thermodynamics and kinetics. Here we demonstrate an innovative phenomenon, i.e., the contact electrification between platinum (Pt) and an n-type gallium arsenide (100) wafer (n-GaAs) can induce interfacial redox reactions. Because of their different work functions, when the Pt electrode comes into contact with n-GaAs, electrons will move from n-GaAs to Pt and form a contact electric field at the Pt/n-GaAs junction until their electron Fermi levels (EF) become equal. In the presence of an electrolyte, the potential of the Pt/electrolyte interface will shift due to the contact electricity and induce the spontaneous reduction of MnO4− anions on the Pt surface. Because the equilibrium of contact electrification is disturbed, electrons will transfer from n-GaAs to Pt through the tunneling effect. Thus, the accumulated positive holes at the n-GaAs/electrolyte interface make n-GaAs dissolve anodically along the Pt/n-GaAs/electrolyte 3-phase interface. Based on this principle, we developed a direct electrochemical nanoimprint lithography method applicable to crystalline semiconductors.
- This article is part of the themed collections: Celebrating a Century of Excellency in Chemistry at Xiamen University and In celebration of Chinese New Year


 Please wait while we load your content...
Please wait while we load your content...