Palladium(0)-catalyzed asymmetric C(sp3)–H arylation using a chiral binol-derived phosphate and an achiral ligand†
Abstract
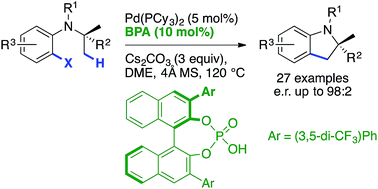
The first efficient palladium(0)-catalyzed enantioselective C(sp3)–H activation reaction using a catalytic chiral base and an achiral phosphine ligand is reported. Fine-tuning the binol-derived phosphoric acid pre-catalyst and the reaction conditions was found to be crucial to achieve high levels of enantioselectivity for a variety of indoline products containing both tri- and tetrasubstituted stereocenters.


 Please wait while we load your content...
Please wait while we load your content...