Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements†
Abstract
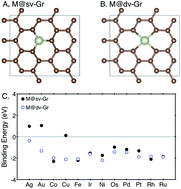
A single-atom catalyst (SAC) has an electronic structure that is very different from its bulk counterparts, and has shown an unexpectedly high specific activity with a significant reduction in noble metal usage for CO oxidation, fuel cell and hydrogen evolution applications, although physical origins of such performance enhancements are still poorly understood. Herein, by means of density functional theory (DFT) calculations, we for the first time investigate the great potential of single atom catalysts for CO2 electroreduction applications. In particular, we study a single transition metal atom anchored on defective graphene with single or double vacancies, denoted M@sv-Gr or M@dv-Gr, where M = Ag, Au, Co, Cu, Fe, Ir, Ni, Os, Pd, Pt, Rh or Ru, as a CO2 reduction catalyst. Many SACs are indeed shown to be highly selective for the CO2 reduction reaction over a competitive H2 evolution reaction due to favorable adsorption of carboxyl (*COOH) or formate (*OCHO) over hydrogen (*H) on the catalysts. On the basis of free energy profiles, we identified several promising candidate materials for different products; Ni@dv-Gr (limiting potential UL = −0.41 V) and Pt@dv-Gr (−0.27 V) for CH3OH production, and Os@dv-Gr (−0.52 V) and Ru@dv-Gr (−0.52 V) for CH4 production. In particular, the Pt@dv-Gr catalyst shows remarkable reduction in the limiting potential for CH3OH production compared to any existing catalysts, synthesized or predicted. To understand the origin of the activity enhancement of SACs, we find that the lack of an atomic ensemble for adsorbate binding and the unique electronic structure of the single atom catalysts as well as orbital interaction play an important role, contributing to binding energies of SACs that deviate considerably from the conventional scaling relation of bulk transition metals.
- This article is part of the themed collections: Most downloaded articles of 2017: Energy and Catalysis and Global Energy Challenges: Hydrogen Energy


 Please wait while we load your content...
Please wait while we load your content...