Co/NHPI-mediated aerobic oxygenation of benzylic C–H bonds in pharmaceutically relevant molecules†
Abstract
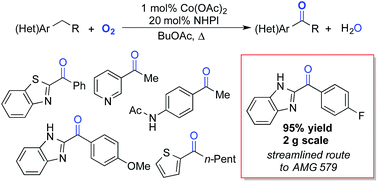
A simple cobalt(II)/N-hydroxyphthalimide catalyst system has been identified for selective conversion of benzylic methylene groups in pharmaceutically relevant (hetero)arenes to the corresponding (hetero)aryl ketones. The radical reaction pathway tolerates electronically diverse benzylic C–H bonds, contrasting recent oxygenation reactions that are initiated by deprotonation of a benzylic C–H bond. The reactions proceed under practical reaction conditions (1 M substrate in BuOAc or EtOAc solvent, 12 h, 90–100 °C), and they tolerate common heterocycles, such as pyridines and imidazoles. A cobalt-free, electrochemical, NHPI-catalyzed oxygenation method overcomes challenges encountered with chelating substrates that inhibit the chemical reaction. The utility of the aerobic oxidation method is showcased in the multigram synthesis of a key intermediate towards a drug candidate (AMG 579) under process-relevant reaction conditions.
- This article is part of the themed collection: Most downloaded articles of 2017: Energy and Catalysis

 Please wait while we load your content...
Please wait while we load your content...