Highly enantioselective metallation–substitution alpha to a chiral nitrile†
Abstract
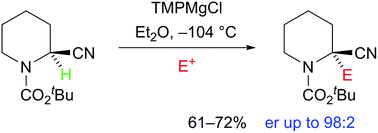
We report the deprotonation of a chiral nitrile and reaction of the resulting chiral organometallic species with a variety of electrophiles to give highly enantiomerically enriched 2-substituted nitrile products. The nitrile was treated with TMPMgCl and the resulting anion, an asymmetric alpha cyano Grignard species, was found to be configurationally stable at low temperature for a short time (half-life several minutes at −104 °C).


 Please wait while we load your content...
Please wait while we load your content...