pH controlled assembly of a self-complementary halogen-bonded dimer†
Abstract
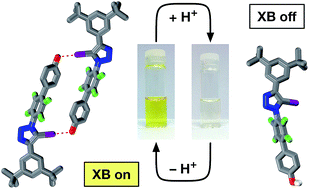
Phenols and their corresponding phenoxide anions can form halogen bonds with neutral iodotriazoles. The strength of these interactions depends critically on the protonation state of the oxygen atom – the interaction of the phenoxide anion is more than an order of magnitude stronger than the corresponding phenol. The assembly of a molecule bearing both an iodotriazole and a phenoxide anion into a self-complementary dimer, stabilised by two halogen bonds between the phenoxide anions and the neutral iodotriazoles has been demonstrated. The corresponding phenol shows no halogen bond mediated assembly either in the solid or in the solution state. This assembly process can be actuated simply by a change in protonation state – treatment of the phenol with one equivalent of base results in deprotonation and assembly of the dimer. The structure of the homodimer formed by the phenoxide-bearing iodotriazole has been determined in the solid state and 19F NMR spectroscopy demonstrates that the assembled dimer persists in solution and that it has significant stability. 19F NMR spectroscopy has also been used to demonstrate that the assembly process is completely reversible.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...