Pyramidalization/twisting of the amide functional group via remote steric congestion triggered by metal coordination†
Abstract
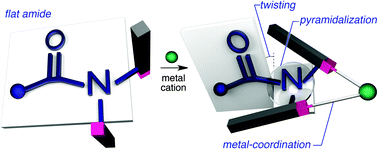
For decades, the planarity of the amide functional group has garnered sustained interest in organic chemistry, enticing chemists to deform its usually characteristic high-fidelity plane. As opposed to the construction of amides that are distorted by imposing rigid covalent bond assemblies, we demonstrate herein the deformation of the amide plane through increased steric bulk in the periphery of the amide moiety, which is induced by coordination to metal cations. A crystallographic analysis revealed that the thus obtained amides exhibit significant pyramidalization and twisting upon coordination to the metals, while the amide functional group remained intact. The observed deformation, which should be attributed to through-space interactions, substantially enhanced the solvolytic cleavage of the amide, providing compelling evidence that temporary crowding in the periphery of the amide functional group may be used to control the reactivity of amides.

 Please wait while we load your content...
Please wait while we load your content...