Copper carbenes alkylate guanine chemoselectively through a substrate directed reaction†
Abstract
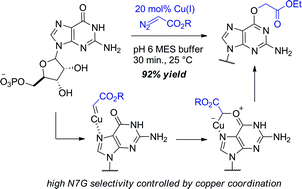
Cu(I) carbenes derived from α-diazocarbonyl compounds lead to selective alkylation of the O6 position in guanine (O6-G) in mono- and oligonucleotides. Only purine-type lactam oxygens are targeted – other types of amides or lactams are poorly reactive under conditions that give smooth alkylation of guanine. Mechanistic studies point to N7G as a directing group that controls selectivity. Given the importance of O6-G adducts in biology and biotechnology we expect that Cu(I)-catalyzed O6-G alkylation will be a broadly used synthetic tool. While the propensity for transition metals to increase redox damage is well-appreciated, our results suggest that transition metals might also increase the vulnerability of nucleic acids to alkylation damage.

 Please wait while we load your content...
Please wait while we load your content...