Magnetic circular dichroism studies of iron(ii) binding to human calprotectin†
Abstract
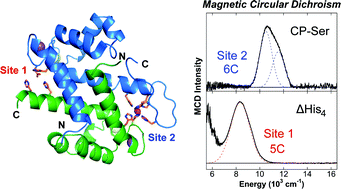
Calprotectin (CP) is an abundant metal-chelating protein involved in host defense, and the ability of human CP to bind Fe(II) in a calcium-dependent manner was recently discovered. In the present study, near-infrared magnetic circular dichroism spectroscopy is employed to investigate the nature of Fe(II) coordination at the two transition-metal-binding sites of CP that are a His3Asp motif (site 1) and a His6 motif (site 2). Upon the addition of sub-stoichiometric Fe(II), a six-coordinate (6C) Fe(II) center associated with site 2 is preferentially formed in the presence of excess Ca(II). This site exhibits an exceptionally large ligand field (10Dq = 11 045 cm−1) for a non-heme Fe(II) protein. Analysis of CP variants lacking residues of the His6 motif supports that CP coordinates Fe(II) at site 2 by employing six His ligands. In the presence of greater than one equiv. of Fe(II) or upon mutation of the His6 motif, the metal ion also binds at site 1 of CP to form a five-coordinate (5C) Fe(II)–His3Asp motif that was previously unidentified in this system. Notably, the introduction of His-to-Ala mutations at the His6 motif results in a mixture of 6C (site 2) and 5C (site 1) signals in the presence of sub-stoichiometric Fe(II). These results are consistent with a reduced Fe(II)-binding affinity of site 2 as more weakly coordinating water-derived ligands complete the 6C site. In the absence of Ca(II), both sites 1 and 2 are occupied upon addition of sub-stoichiometric Fe(II), and a stronger ligand field is observed for the 5C site. These spectroscopic studies provide further evaluation of a unique non-heme Fe(II)–His6 site for metalloproteins and support the notion that Ca(II) ions influence the Fe(II)-binding properties of CP.

 Please wait while we load your content...
Please wait while we load your content...