Fluorinative ring-opening of cyclopropanes by hypervalent iodine reagents. An efficient method for 1,3-oxyfluorination and 1,3-difluorination†
Abstract
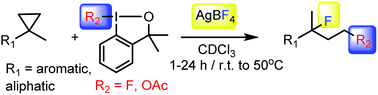
A new method is presented for 1,3-difluorination and 1,3-oxyfluorination reactions. The process is based on iodonium mediated opening of 1,1-disubstituted cyclopropanes. The reaction proceeds with high chemo- and regioselectivity under mild reaction conditions typically at room temperature in a couple of hours. The reaction probably occurs via electrophilic ring-opening of cyclopropanes.

 Please wait while we load your content...
Please wait while we load your content...