Geminal bis-borane formation by borane Lewis acid induced cyclopropyl rearrangement and its frustrated Lewis pair reaction with carbon dioxide†
Abstract
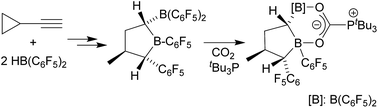
Cyclopropylacetylene reacts with two molar equivalents of Piers' borane [HB(C6F5)2] under mild conditions by an addition/rearrangement sequence with cyclopropyl ring opening to give a mixture of two α-B(C6F5)2 substituted tetrahydroboroles. This compound forms an active frustrated Lewis pair with PtBu3 that heterolytically splits dihydrogen and adds carbon dioxide as a geminal chelate bis-boryl component. The respective reactions of the two-fold HB(C6F5)2 addition to Ph-CH2CH2C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH were studied as a geminal Lewis acid reference. Most of the products were characterized by X-ray diffraction.
CH were studied as a geminal Lewis acid reference. Most of the products were characterized by X-ray diffraction.

 Please wait while we load your content...
Please wait while we load your content...