Low picomolar, instrument-free visual detection of mercury and silver ions using low-cost programmable nanoprobes
Abstract
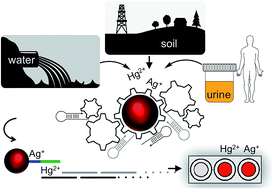
The EPA's recommended maximum allowable level of inorganic mercury in drinking water is 2 ppb (10 nM). To our knowledge, the most sensitive colorimetric mercury sensor reported to date has a limit of detection (LOD) of 800 pM. Here, we report an instrument-free and highly practical colorimetric methodology, which enables detection of as low as 2 ppt (10 pM) of mercury and/or silver ions with the naked eye using a gold nanoprobe. Synthesis of the nanoprobe costs less than $1.42, which is enough to perform 200 tests in a microplate; less than a penny for each test. We have demonstrated the detection of inorganic mercury from water, soil and urine samples. The assay takes about four hours and the color change is observed within minutes after the addition of the last required element of the assay. The nanoprobe is highly programmable which allows for the detection of mercury and/or silver ions separately or simultaneously by changing only a single parameter of the assay. This highly sensitive approach for the visual detection relies on the combination of the signal amplification features of the hybridization chain reaction with the plasmonic properties of the gold nanoparticles. Considering that heavy metal ion contamination of natural resources is a major challenge and routine environmental monitoring is needed, yet time-consuming, this colorimetric approach may be instrumental for on-site heavy metal ion detection. Since the color transition can be measured in a variety of formats including using the naked eye, a simple UV-Vis spectrophotometer, or recording using mobile phone apps for future directions, our cost-efficient assay and method have the potential to be translated into the field.

 Please wait while we load your content...
Please wait while we load your content...