Insights into ClpXP proteolysis: heterooligomerization and partial deactivation enhance chaperone affinity and substrate turnover in Listeria monocytogenes†
Abstract
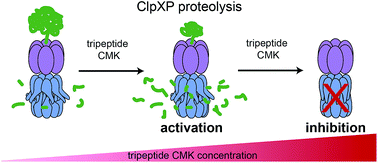
Caseinolytic proteases (ClpP) are important for recognition and controlled degradation of damaged proteins. While the majority of bacterial organisms utilize only a single ClpP, Listeria monocytogenes expresses two isoforms (LmClpP1 and LmClpP2). LmClpPs assemble into either a LmClpP2 homocomplex or a LmClpP1/2 heterooligomeric complex. The heterocomplex in association with the chaperone ClpX, exhibits a boost in proteolytic activity for unknown reasons. Here, we use a combined chemical and biochemical strategy to unravel two activation principles of LmClpPs. First, determination of apparent affinity constants revealed a 7-fold elevated binding affinity between the LmClpP1/2 heterocomplex and ClpX, compared to homooligomeric LmClpP2. This tighter interaction favors the formation of the proteolytically active complex between LmClpX and LmClpP1/2 and thereby accelerating the overall turnover. Second, screening a diverse library of fluorescent labeled peptides and proteins with various ClpP mutants allowed the individual analysis of substrate preferences for both isoforms within the heterocomplex. In addition to Leu and Met, LmClpP2 preferred a long aliphatic chain (2-Aoc) in the P1 position for cleavage. Strikingly, design and synthesis of a corresponding 2-Aoc chloromethyl ketone inhibitor resulted in stimulation of proteolysis by 160% when LmClpP2 was partially alkylated on 20% of the active sites. Determination of apparent affinity constants also revealed an elevated complex stability between partially modified LmClpP2 and the cognate chaperone LmClpX. Thus, the stimulation of proteolysis through enhanced binding to the chaperone seems to be a characteristic feature of LmClpPs.


 Please wait while we load your content...
Please wait while we load your content...