Molecular titanium nitrides: nucleophiles unleashed†
Abstract
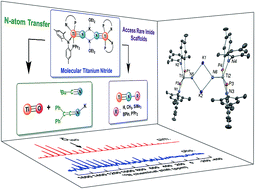
In this contribution we present reactivity studies of a rare example of a titanium salt, in the form of [μ2-K(OEt2)]2[(PN)2Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N]2 (1) (PN− = N-(2-(diisopropylphosphino)-4-methylphenyl)-2,4,6-trimethylanilide) to produce a series of imide moieties including rare examples such as methylimido, borylimido, phosphonylimido, and a parent imido. For the latter, using various weak acids allowed us to narrow the pKa range of the NH group in (PN)2Ti
N]2 (1) (PN− = N-(2-(diisopropylphosphino)-4-methylphenyl)-2,4,6-trimethylanilide) to produce a series of imide moieties including rare examples such as methylimido, borylimido, phosphonylimido, and a parent imido. For the latter, using various weak acids allowed us to narrow the pKa range of the NH group in (PN)2Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) NH to be between 26–36. Complex 1 could be produced by a reductively promoted elimination of N2 from the azide precursor (PN)2TiN3, whereas reductive splitting of N2 could not be achieved using the complex (PN)2Ti
NH to be between 26–36. Complex 1 could be produced by a reductively promoted elimination of N2 from the azide precursor (PN)2TiN3, whereas reductive splitting of N2 could not be achieved using the complex (PN)2Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ti(PN)2 (2) and a strong reductant. Complete N-atom transfer reactions could also be observed when 1 was treated with ClC(O)tBu and OCCPh2 to form NCtBu and KNCCPh2, respectively, along with the terminal oxo complex (PN)2Ti
Ti(PN)2 (2) and a strong reductant. Complete N-atom transfer reactions could also be observed when 1 was treated with ClC(O)tBu and OCCPh2 to form NCtBu and KNCCPh2, respectively, along with the terminal oxo complex (PN)2Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O, which was also characterized. A combination of solid state 15N NMR (MAS) and theoretical studies allowed us to understand the shielding effect of the counter cation in dimer 1, the monomer [K(18-crown-6)][(PN)2Ti
O, which was also characterized. A combination of solid state 15N NMR (MAS) and theoretical studies allowed us to understand the shielding effect of the counter cation in dimer 1, the monomer [K(18-crown-6)][(PN)2Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N], and the discrete salt [K(2,2,2-Kryptofix)][(PN)2Ti
N], and the discrete salt [K(2,2,2-Kryptofix)][(PN)2Ti![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N] as well as the origin of the highly downfield 15N NMR resonance when shifting from dimer to monomer to a terminal nitride (discrete salt). The upfield shift of 15Nnitride resonance in the 15N NMR spectrum was found to be linked to the K+ induced electronic structural change of the titanium-nitride functionality by using a combination of MO analysis and quantum chemical analysis of the corresponding shielding tensors.
N] as well as the origin of the highly downfield 15N NMR resonance when shifting from dimer to monomer to a terminal nitride (discrete salt). The upfield shift of 15Nnitride resonance in the 15N NMR spectrum was found to be linked to the K+ induced electronic structural change of the titanium-nitride functionality by using a combination of MO analysis and quantum chemical analysis of the corresponding shielding tensors.


 Please wait while we load your content...
Please wait while we load your content...