Bimetallic nanosized solids with acid and redox properties for catalytic activation of C–C and C–H bonds†
Abstract
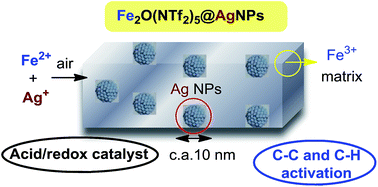
A new approach is presented to form self-supported bimetallic nanosized solids with acid and redox catalytic properties. They are water-, air- and H2-stable, and are able to activate demanding C–C and C–H reactions. A detailed mechanistic study on the formation of the Ag–Fe bimetallic system shows that a rapid redox-coupled sequence between Ag+, O2 (air) and Fe2+ occurs, giving monodisperse Ag nanoparticles supported by O-bridged diatomic Fe3+ triflimides. The system can be expanded to Ag nanoparticles embedded within a matrix of Cu2+, Bi3+ and Yb3+ triflimide.


 Please wait while we load your content...
Please wait while we load your content...