Polymorphism at 129 dictates metastable conformations of the human prion protein N-terminal β-sheet†
Abstract
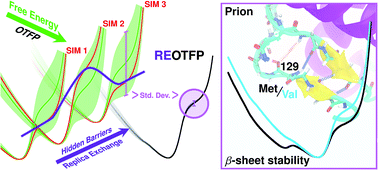
We study the thermodynamic stability of the native state of the human prion protein using a new free-energy method, replica-exchange on-the-fly parameterization. This method is designed to overcome hidden-variable sampling limitations to yield nearly error-free free-energy profiles along a conformational coordinate. We confirm that all four (M129V, D178N) polymorphs have a ground-state conformation with three intact β-sheet hydrogen bonds. Additionally, they are observed to have distinct metastabilities determined by the side-chain at position 129. We rationalize these findings with reference to the prion “strain” hypothesis, which links the variety of transmissible spongiform encephalopathy phenotypes to conformationally distinct infectious prion forms and classifies distinct phenotypes of sporadic Creutzfeldt-Jakob disease based solely on the 129 polymorphism. Because such metastable structures are not easily observed in structural experiments, our approach could potentially provide new insights into the conformational origins of prion diseases and other pathologies arising from protein misfolding and aggregation.


 Please wait while we load your content...
Please wait while we load your content...