C(sp3)–C(sp2) cross-coupling of alkylsilicates with borylated aryl bromides – an iterative platform to alkylated aryl- and heteroaryl boronates†
Abstract
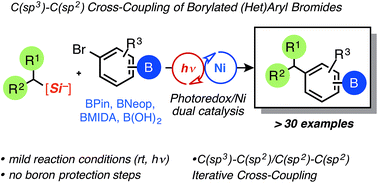
The attractive field of iterative cross-coupling has seen numerous advances, although almost exclusively in the union of sp2-hybridized partners. Conspicuously absent from this useful synthetic manifold is the inclusion of sp3-hybridized pronucleophiles that can undergo transmetalation under mild conditions. Described here is the use of primary and secondary ammonium alkylsilicates, which undergo facile C(sp3)–C(sp2) cross-coupling with borylated aryl bromide partners under photoredox/nickel dual catalysis conditions. This operationally simple procedure allows the production of alkylated small molecules possessing boronate ester (BPin, Bneopentyl, BMIDA) functional handles. Because of the extremely mild reaction conditions and the innocuous byproduct generated upon fragmentative oxidation of silicates, the corresponding borylated compounds were isolated in good to excellent yields. Aryl bromides bearing unprotected boronic acids are also generally tolerated for the first time and prove useful in multistep syntheses. Unlike many previously reported photoredox/Ni dual cross-couplings, the C(sp3)–C(sp2) bonds were forged using a transition metal-free photocatalyst, allowing a substantial increase in sustainability as well as a cost reduction. Because the developed Ni-catalyzed cross-coupling does not require discrete boron speciation control, as in many popular orthogonal Pd-based methods, this protocol represents a significant advance in atom- and step-economy.

 Please wait while we load your content...
Please wait while we load your content...